|
Identification
of risks associated with arrhythmic death
remains a great challenge.
Although great
progress has been made in the prevention of
cardiovascular diseases, the mortality remains high
for patients after myocardial infarction or heart
failure who often die without previous symptoms.
Almost half of all cardiovascular deaths can be
attributed to this unexpected and not yet predictable
cause. Sudden cardiac death (SCD) as a consequence of
coronary heart disease has been rated the most
important cause of death in the adult population in
industrialized societies.
Mechanisms
of action of omega-3 fatty acid ethyl esters in
heart dysfunction.
Myocardial infarction and severity of coronary
artery disease are associated with a higher risk of
SCD. In fact, coronary artery disease is known to be
present in about 80% of patients who suffer from SCD,
whereby the proportion of deaths that are sudden is
higher in mild to moderate heart failure (HF). Like in
the MERIT-HF study, the incidence of SCD was 64% in
New York Heart Association (NYHA) functional class II,
59% in class III and was reduced to 33% in class IV.
Preventing SCD is thus particularly important for
patients during early progression of heart failure
when their pump function is still associated with good
quality of life.
Irrespective of the underlying pathophysiology
(ischemic or non-ischemic heart failure), SCD is very
often caused by ventricular tachycardia that
degenerates to ventricular fibrillation. However, the
majority of patients who die suddenly during
progression of heart failure cannot be identified by
current risk stratification. Since the protective
action of implantable cardioverter-defibrillators
(ICDs) and highly purified EPA+DHA ethyl esters
(Omacor) depends on the risk of SCD in a given patient
population, efforts should be made to better
understand the pathophysiology of HF. Particularly,
mechanisms inferred from depressed pump function often
monitored by reduced ejection fraction (EF) remain
unresolved. EF is a composite of various physiological
factors with major contributions from:
i. Remodeling of the hypertrophied cardiocyte and the
extracellular matrix leading to depressed
"contractility". While fibrosis has long been alleged
as contributing factor to malignant arrhythmias, the
dysregulated gene expression of hypertrophied
cardiocytes (e.g. lack in upregulation of the
sarcoplasmic reticulum Ca2+ pump gene, SERCA2,
partially corrected by the FOXIB/PPARalpha
etomoxir (1,2)) leading to diastolic Ca2+
overload and electric instability has only recently
been explored and is not targeted by current therapy.
Thus, SCD not only occurs in patients with systolic
heart failure but is also not uncommon in patients
with diastolic heart failure. Based on the many
defects in cardiocyte gene expression and
extracellular matrix remodeling, left ventricular
hypertrophy (LVH) emerged as a major risk for SCD. LVH
occurs as a result of a hemodynamic overload (e.g.
surviving myocardium after MI or hypertension). Thus,
the anti-arrhythmogenic intervention with Omacor is
expected to have a protective action not only in
post-MI but also during progression of HF associated
with LVH irrespective of its etiology.
ii. The “compensated” stage of concentric hypertrophy
can often not be maintained and enlargement of the LV
occurs, i.e. dilatation. A deleterious consequence is
the rise in wall stress (according to LaPlace, wall
stress is increased by an increase in intraventricular
pressure and radius and decreased when wall thickness
is increased, i.e. as in LVH). Wall stress reflects
the tension a cardiocyte has to develop during
systole. Since the LVH response is limited e.g. by
coronary blood and energy supply, LV dilatation is
often not adequately compensated by LVH and a rise in
wall stress ensues. A high wall stress has various
adverse effects including an increased opening
probability of stretch-activated cation channels which
raises the risk of ectopic activity. A reduction of
diastolic Ca2+ through partial inhibition of the late
and fast Na+ current and the L-type Ca2+ current by
EPA+DHA would, therefore, be useful. Wall stress is
also the major determinant of oxygen consumption of
the heart and, thereby, can aggravate latent ischemia.
Myocardial stretch can also trigger
afterdepolarisations and extrasystoles. Furthermore,
the conduction system is often impaired by mechanical
stretch resulting in ventricular dysynchrony (a target
for resynchronization
therapy). While wall stress has been calculated
in the past from echocardiography data, it was only
recently shown by Alter et al. that the actual wall
stress is underestimated and that only volumetric data
derived from cardiac magnetic resonance imaging (MRI)
permit accurate wall stress assessment (3). The
MRI-based wall stress was correlated with raised serum
brain natriuretic peptide (BNP), a marker of
cardiocyte stretch (4). MRI-based wall stress is
expected to have a greater specificity compared with
BNP which nonetheless has a positive predictive value
for SCD. The MRI-based wall stress calculation
provides the long-sought tool for monitoring
progression of heart failure in terms of LV dilatation
and LVH (5). It became also possible to predict the LV
afterload reduction required for returning wall stress
into the normal range. Using “isostress” curves, the
systolic pressure had, however, to be reduced in some
patients to a level which is too low for maintaining
adequate organ perfusion (3).
iii. a reduced endogenous production of long-chain
desaturated fatty acids by an altered activity of
delta-6 and delta-5 desaturase. By this mechanism,
EPA+DHA is expected to be reduced which can be viewed
as “lipid remodelling”. Particularly crucial is in
this respect marked cardiac dilatation. (Rupp et al.
unpublished). Any protective action of Omacor or also
ICDs will depend on the impact of these factors,
whereby an increased wall stress appears to be the
most crucial one. Thus, after myocardial infarction,
LV dilatation occurs in about 20-30% of patients which
appears not to be adequately compensated by LVH
resulting most probably in a markedly raised wall
stress and thus high risk of SCD. It would thus also
be of great interest to examine whether in HF patients
with high wall stress the benefit of ICDs is greater
and whether a high wall stress can account for the
greater benefit of ICDs in ischemic versus
non-ischemic HF. It is thus proposed to assess
anti-arrhythmogenic effects of EPA+DHA ethyl esters in
patients in terms of MRI-based wall stress and the
“EPA+DHA level” (6).
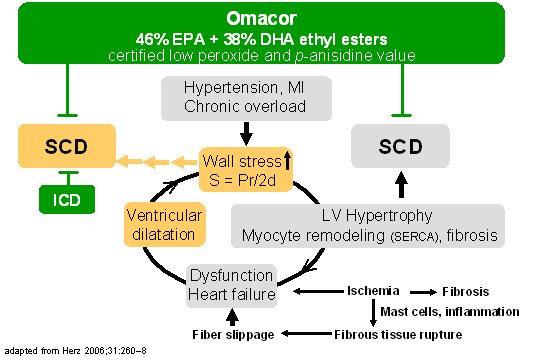
Schematic
presentation of pathophysiological events raising the
risk of SCD. Various vicious cycles raise the risk of
SCD and HF. The risk of SCD can be reduced by
prophylactic ICD implantation or by prescription
omega-3 fatty acids (based on GISSI-P, Omacor) where a
low peroxide and p-anisidine value (marker of adverse
oxidation of EPA and DHA) is certified which can be
monitored also by SPME-GC/ion trap MS in terms of
volatile aldehydes/ketones (pungent “fishy” odour;
Rupp, unpublished). The ICD indication is given here
for the dilated heart of high wall stress where EF is
expected to be <35%. However, SCD risk is also high
in patients with EF in between 35% and 50% (mild to
moderate heart failure) which is considered an
important therapeutic target for Omacor.
EPA+DHA
ethyl esters (Omacor) in heart failure
The value of
adding 1g/day EPA-DHA ethyl esters (Omacor) to
standard therapy for heart failure has been
established by the results of the recent GISSI-HF
trial (7). This randomized, double-blind,
placebo-controlled trial, conducted at 357 cardiology
or internal medicine centres in Italy under the
direction of the GISSI group recruited 7046 patients
with heart failure. Omacor therapy was associated with
statistically significant benefits on both the
co-primary endpoints: time to death from any cause –
the adjusted hazard ratio was 0.91 (95.5% CI
0.833–0.998; P = 0.041). For time to all-cause
mortality or admission to hospital for any
cardiovascular reason the adjusted hazard ratio was
0.92 (99% CI 0.849–0.999; P = 0.009). The effects were
consistent across a wide range of prespecified
subgroups, including EF > or <40%, aetiology of
heart failure (ischemic vs non-ischemic), baseline
NYHA grade, diabetes at baseline or age.
The effects of Omacor on the GISSI-HF primary
endpoints can also be expressed as the number of
patients that have to be treated for the time of the
follow-up to prevent one endpoint event, i.e. number-needed-to-treat
(NNT). For all-cause mortality, NNT for Omacor was 56;
for all-cause mortality or hospitalization for a
cardiovascular cause, the NNT was 44. Said in other
words, when 1000 patients are treated with Omacor for
~4 years, 18 lives were saved and 17 cardiovascular
hospitalizations were prevented.
It has been pointed out by Gregg C. Fonarow
(Ahmanson-UCLA
Cardiomyopathy Center, Los Angeles) in the Comment
"Statins and n-3 fatty acid supplementation in heart
failure" to the GISSI-HF study: "...Whilst questions
remain about mechanisms of action, optimum dosing, and
formulation, supplementation with n-3 polyunsaturated
fatty acids should join the short list of
evidence-based life-prolonging therapies for heart
failure." (Lancet. Online 2008 Aug 29)
Evidence-based
therapies
for systolic heart failure (from G C Fonarow, Lancet. Online
2008 Aug 29)
Therapy
|
Relative-risk reduction in
all-cause mortality
|
Angiotensin-converting-enzyme
inhibitors or angiotensin-receptor antagonists
|
17–25%
|
β blockers
|
34–35%
|
Aldosterone antagonists*
|
15–30%
|
Hydralazine-isosorbide dinitrate*
|
43%
|
Implantable cardioverter
defibrillator*
|
23%
|
Cardiac resynchronisation therapy*
|
36%
|
n-3
polyunsaturated fatty acid supplementation
|
9%
|
*For patients
with specific indications.
The
results of GISSI-HF mandate, therefore, in our opinion
the use of Omacor 1 g/day in heart failure patients.
Heart failure guidelines should be amended to reflect
this fact.
It shoud be pointed out that a specific medication was
used in the GISSI trials, i.e. Omacor and it is
unscientific to infer that regular fish oil capsules
could be a substitute. It is also not justified to
simplify this medication by referring to it as "n-3
polyunsaturated fatty acid supplementation". It is
hoped that this aspect will be corrected in the
guidelines. On the other hand, manufacturers of fish
oils are encouraged to initiate trials and to assess
efficacy or lack of efficacy of their preparations.
1.
Turcani M, Rupp H. Etomoxir improves left
ventricular performance of pressure-overloaded rat
heart. Circulation. 1997;96:3681-3686.
2. Rupp H, Rupp TP, Maisch B. Fatty acid oxidation
inhibition with PPARalpha activation
(FOXIB/PPARalpha) for normalizing gene expression in
heart failure? Cardiovasc Res. 2005;66:423-426.
3. Alter P, Rupp H, Rominger MB, Vollrath A, Czerny
F, Figiel JH, Adams P, Stoll F, Klose KJ, Maisch B.
B-type natriuretic peptide and wall stress in
dilated human heart. Mol Cell Biochem.
2008;314:179-191.
4. Alter P, Rupp H, Rominger MB, Vollrath A, Czerny
F, Klose KJ, Maisch B. Relation of B-type
natriuretic peptide to left ventricular wall stress
as assessed by cardiac magnetic resonance imaging in
patients with dilated cardiomyopathy. Can J Physiol
Pharmacol. 2007;85:790-9.
5. Alter P, Rupp H, Rominger MB, Klose KJ, Maisch B.
A new methodological approach to assess cardiac work
by pressure-volume and stress-length relations in
patients with aortic valve stenosis and dilated
cardiomyopathy. Pflugers Arch. 2008;455:627-36.
6. Rupp H, Wagner D, Rupp T, Schulte LM, Maisch B.
Risk stratification by the "EPA+DHA level" and the
"EPA/AA ratio" focus on anti-inflammatory and
antiarrhythmogenic effects of long-chain omega-3
fatty acids. Herz. 2004;29:673-85.
7.GISSI-HF
Investigators. Effect of n-3
polyunsaturated fatty acids in patients with
chronic heart failure (the GISSI-HF trial): a
randomised, double-blind, placebo-controlled
trial. Lancet.
2008;372:1223-1230.
Webcast:
New
perspectives
for
an
evidence-based therapy with omega-3 fatty acid
ethyl esters
Malignant arrhythmias and sudden
cardiac death in myocardial infarction.
The major pathophysiological cause of SCD is seen in
the electrical instability of the infarct zone and the
non infarcted muscle. Because of the loss of
contractile tissue, the surviving hypertrophied
myocardium is subjected to various adverse
neuroendocrine influences. A consequence is an
unfavorable cellular and molecular restructuring of
the extracellular matrix and the cardiomyocyte. The
fibrosis also worsens coronary blood supply and thus
amplifies the risk of reinfarction. In approximately
one third of post-MI patients, dilatation of the left
ventricle occurs. Since dilated hearts exhibit an
increased wall stress favouring also the opening of
stretch-activated ion channels (1), the resulting
electrical instability contributes to the increased
risk of ventricular tachyarrhythmias. All these
adverse processes contribute to worsening of pump
function which is reflected in a reduced EF which is
the most often used predictor of malignant
arrhythmias. Conventional therapy is, however, only
partially directed against mechanisms which promote
electrical instability. The remaining electrical
instability after MI can be counteracted by modulating
the activity of membrane ion channels through
incorporation of long-chain omega-3 fatty acids,
particularly DHA, in their microenvironment. The
administration of Omacor should, therefore, also be
seen in the context of the implantable cardioverter
defibrillator (ICD) therapy
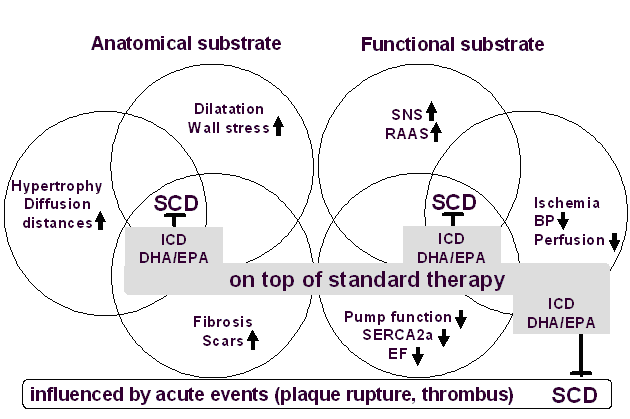
SCD
prevention
by ICDs and Omacor
ICDs are implanted in patients with EF <35-40,
whereby the number of patients needed to treat (NNT)
to prevent a sudden death was 11 in MADIT-II and 14 in
SCD-HeFT. Only in MADIT-I, the NNT was 4 when
EF<35% was combined with the presence of
non-sustained ventricular tachycardia. Nonetheless, a
substantial proportion of patients will die suddenly
despite ICD implantation (2). ICD therapy is
associated with a relative risk reduction of SCD of
approximately 60%, far less than the greater than 90%
efficacy that many expect (2). Reducing the incidence
of ICD-unresponsive SCD would substantially improve
survival and cost-effectiveness related to ICD
therapy. Alternatives for reducing SCD risk are thus
required. Pharmacological interventions proved not to
be successful. Proarrhythmic and negative inotropic
effects of class Ia and Ic antiarrhythmics are more
pronounced during progression of heart failure. The
class III antiarrhythmic D-sotalol which lacks
beta-blocking action even increased mortality in
post-MI patients with reduced pump function (3). For
amiodarone, no significant mortality reduction was
observed in chronic HF or after MI and on prognostic
terms represents, therefore, no alternative for an
ICD. The need of alternatives for SCD prevention is
demonstrated also by the fact that in post-MI patients
the risk of SCD is increased already at EF<50%. In
the GISSI-Prevenzione study, 86% of patients had
EF>40% and only 2.5% EF<30%. In the light of ICD
guidelines, one would therefore expect only a very
limited effect for interventions targeting SCD. This
was, however, not the case as seen in the comparison
of trials with ICD therapy (2) and the
GISSI-Prevenzione study (4-6) with Omacor
administration.
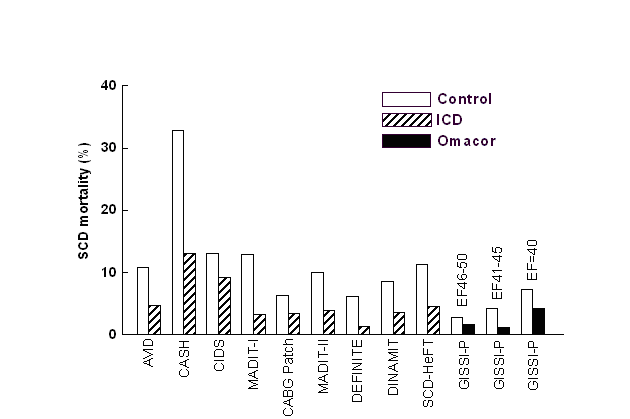
Anti-arrhythmogenic effects of DHA+EPA ethyl esters
In the studies of the GISSI group, 1g omega-3 fatty
acids was administered, whereby the relevance of the
particular formulation has been underrated
particularly in guidelines
i. Ethyl esters but not triglycerides commonly present
in fish oils were used. Ethyl esters result in a
retarded and sustained uptake of DHA and EPA. After
intestinal absorption, long-chain fatty acids reach
the coronaries via the thoracic duct and bypass the
liver (contrary to amino acids and sugars). It appears
that ethyl esters have the advantage of providing a
sustained increase in lymphe DHA and EPA levels (7)
which are expected to contribute to the critical rise
in DHA and EPA required for the antiarrhythmogenic
action.
ii. Omacor used in the GISSI trials contains min 84%
of the long-chain omega-3 fatty acids DHA and EPA,
whereby the ratio of DHA:EPA was 38:46%. An exchange
of DHA for EPA or short-chain fatty acids
(alpha-linolenic acid) is expected to reduce the
antiarrhythmogenic action. In rats with low dose
intake of omega-3 fatty acids, DHA but not EPA
inhibited ischemia-induced cardiac arrhythmias (8). In
the Japan EPA lipid intervention study (JELIS),
hypercholesterolaemic patients were treated with daily
1.8g EPA (9). While a 19% relative reduction in major
coronary events occurred, the (low) risk of SCD was
not reduced further. One should, therefore, not refer
in guidelines to 1g omega-3 fatty acids in general
when referring to the GISSI trials but to specify the
DHA:EPA ratio and to point out that ethyl esters were
used. To demonstrate that Omacor differs markedly from
regular fish oil preparations, representative gas
chromatograms of the constituent fatty acids are
given. The high concentration of EPA and DHA and the
virtual absence of saturated and omega-6 fatty acids
can be achieved only by transesterification of fish
oils with ethanol resulting in ethyl esters with
subsequent purification of the respective DHA and EPA
ethyl esters.
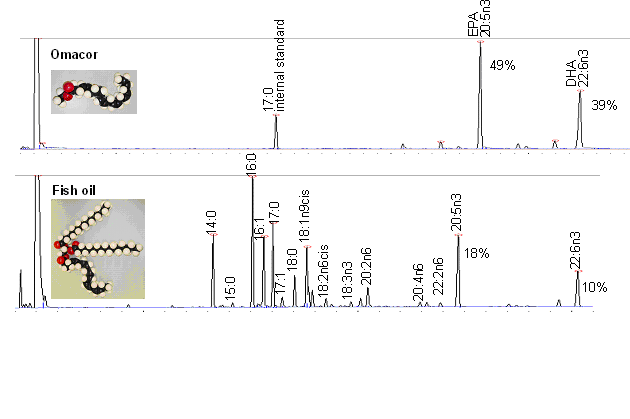
iii. During preparation of ethyl esters more
purification steps are involved than in the extraction
of triglycerides present in fish oils which is
expected to reduce the contamination particularly with
methyl mercury which has been associated with an
increased risk of MI (10). Body mercury was correlated
with omega-3 fatty acids, indicating that omega-3
fatty acids were derived from fish or fish oils
contaminated with mercury. It appears, therefore,
mandatory to use in post-MI patients DHA+EPA
preparations with a minimum of methyl mercury and
other environmental pollutants such as PCBs and
dioxins. Work is ongoing to determine such pollutants
in various DHA+EPA preparations and to assess whether
they influence the incidence of dilative
cardiomyopathy.
iv. In post-MI patients on standard care
(anti-platelet drug, beta-blocker, ACE-inhibitor,
statin) a preparation with 1g DHA+EPA in 1 capsule
(Omacor) is required. In a number of non positive,
i.e. "neutral" studies on patients with ICD (sometimes
mislabeled as “negative studies”), the number of
capsules was higher than in the GISSI trials which was
associated with poor patient compliance. In the Study
on Omega-3 Fatty Acids and Ventricular Arrhythmia
(SOFA) by Brouwer et al. (7), ICD patients were
enrolled for assessing the effect of 2 g of "purified
fish oil" (4 capsules/day) vs. placebo (olive oil) on
life-threatening arrhythmias. Judged by capsule count,
76% of patients took more than 80% of the fish oil
capsules. The primary endpoint (appropriate ICD
intervention for ventricular tachycardia or
fibrillation or all-cause death) occurred in 30% of
patients taking fish oil vs. 33% patients taking
placebo (not significant difference). In the study by
Leaf et al. (11), ICD patients were randomized to 2.6g
EPA and DHA ethyl ester (daily four 1g capsules) or
olive oil as placebo for 12 months. Why in this study
capsules with only 65% DHA+EPA instead of 84% as in
the case of Omacor and the trials of the GISSI group
were used, remains intriguing. Compliance with the
double-blind treatment was similar in the two groups;
however, the noncompliance rate was high (35% of all
enrollees). The primary end point, time to first ICD
event for ventricular tachycardia or fibrillation
confirmed by stored ECG or death from any cause was
borderline significant (risk reduction of 28%;
P=0.057). For those who stayed on protocol for at
least 11 months, the antiarrhythmic benefit of DHA+EPA
ethyl esters was improved for those with confirmed
events (risk reduction of 38%; P=0.034). This study
also argues against the use of capsules with a lower
DHA and EPA content, e.g. the approximately 30%
DHA+EPA of regular fish oil. The known low compliance
with multiple capsule intake (e.g. 32% permanent
noncompliance for beta-blocker use in COMET (12)) was
also one of the reasons for the production of ethyl
esters using transesterification of fish oil
triglycerides resulting in the highly concentrated
preparation of Omacor. The critical impact of
noncompliance can be derived also from the observation
that already 2 days after Omacor intake, the serum
DHA+EPA level has reached again baseline (Rupp,
unpublished). Thus, DHA+EPA has to be released from
membranes during an ischemic event which would
obviously be more pronounced in MI than in HF or even
an ICD event.
In sum,
although great progress has been made in elucidating
the antiarrhythmogenic action of DHA+EPA and the
trials of the GISSI group have provided clear evidence
on the clinical effectiveness in arrhythmic event
prevention also when compared with ICD therapy, one
should be very careful when referring to the active
ingredients of Omacor which are ethyl esters and not
triglycerides as in fish oils. It is not justified to
extrapolate to omega-3 fatty acids in general
implicating the use of short-chain omega-3 fatty acids
such as alpha-linolenic acid. Also substitution of EPA
for DHA is expected to result in a different
therapeutic profile. To avoid further confusion, it is
suggested to clearly specify the actual ingredients
particularly in guidelines and to adhere to the
accepted chemical nomenclature.
(1) Franz MR, Cima R, Wang D, Profitt D, Kurz
R. Electrophysiological effects of myocardial
stretch and mechanical determinants of
stretch-activated arrhythmias. Circulation 1992;
86:968-978.
(2) Anderson KP. Sudden cardiac death unresponsive
to implantable defibrillator therapy: an urgent
target for clinicians, industry and government. J
Interv Card Electrophysiol 2005; 14:71-78.
(3) Doggrell SA, Brown L. D-Sotalol: death by the
SWORD or deserving of further consideration for
clinical use? Expert Opin Investig Drugs 2000;
9:1625-1634.
(4) GISSI-Prevenzione Investigators. Dietary
supplementation with n-3 polyunsaturated fatty acids
and vitamin E after myocardial infarction: results
of the GISSI- Prevenzione trial. Gruppo Italiano per
lo Studio della Sopravvivenza nell'Infarto
miocardico. Lancet 1999; 354:447-55.
(5) Marchioli R, Avanzini F, Barzi F, Chieffo C, Di
Castelnuovo A, Franzosi MG, Geraci E, Maggioni AP,
Marfisi RM, Mininni N, Nicolosi GL, Santini M,
Schweiger C, Tavazzi L, Tognoni G, Valagussa F.
Assessment of absolute risk of death after
myocardial infarction by use of multiple-risk-factor
assessment equations: GISSI- Prevenzione mortality
risk chart. Eur Heart J 2001; 22:2085-103.
(6) Marchioli R, Barzi F, Bomba E, Chieffo C, Di
Gregorio D, Di Mascio R, Franzosi MG, Geraci E,
Levantesi G, Maggioni AP, Mantini L, Marfisi RM,
Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M,
Schweiger C, Tavazzi L, Tognoni G, Tucci C,
Valagussa F. Early protection against sudden death
by n-3 polyunsaturated fatty acids after myocardial
infarction: time-course analysis of the results of
the Gruppo Italiano per lo Studio della
Sopravvivenza nell'Infarto Miocardico
(GISSI)-Prevenzione. Circulation 2002;
105:1897-1903.
(7) Rupp H, Rupp TP, Wagner D, Alter P, Maisch B.
Microdetermination of fatty acids by gas
chromatography and cardiovascular risk
stratification by the "EPA+DHA level". Herz 2006; 31
(suppl 3):30-49.
(8) McLennan P, Howe P, Abeywardena M, Muggli R,
Raederstorff D, Mano M, Rayner T, Head R. The
cardiovascular protective role of docosahexaenoic
acid. Eur J Pharmacol 1996; 300:83-89.
(9) Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y,
Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H,
Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T,
Shimada K, Shirato K. Effects of eicosapentaenoic
acid on major coronary events in
hypercholesterolaemic patients (JELIS): a randomised
open-label, blinded endpoint analysis. Lancet 2007;
369:1090-1098.
(10) Guallar E, Sanz-Gallardo MI, van't Veer P, Bode
P, Aro A, Gomez-Aracena J, Kark JD, Riemersma RA,
Martin-Moreno JM, Kok FJ. Mercury, fish oils, and
the risk of myocardial infarction. N Engl J Med
2002; 347:1747-1754.
(11) Leaf A, Albert CM, Josephson M, Steinhaus D,
Kluger J, Kang JX, Cox B, Zhang H, Schoenfeld D.
Prevention of fatal arrhythmias in high-risk
subjects by fish oil n-3 fatty acid intake.
Circulation 2005; 112:2762-2768.
(12) Poole-Wilson PA, Swedberg K, Cleland JG, Di
Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger
B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A,
Skene A. Comparison of carvedilol and metoprolol on
clinical outcomes in patients with chronic heart
failure in the Carvedilol Or Metoprolol European
Trial (COMET): randomised controlled trial. Lancet
2003; 362:7-13.
How does EPA and DHA work?
Recent emphasis has been placed on malignant
arrhythmia risks associated with a low abundance of
EPA and DHA in the body. Since free acids of EPA and
DHA are required for most of their biological effects
including their antiarrhythmogenic action, it appears
essential not only to build up stores in the body for
their release, but also to provide a sustained uptake
of EPA and DHA in the form of ethyl esters rather than
dietary triglycerides which are present in fish or
fish oils.
The triglyceride on the left contains 2 saturated
fatty acids (C16:0, palmitic acid) and a long-chain
polyunsaturated omega-3 fatty acid (C20:5, EPA,
eicosapentaenoic acid, at the bottom) which have a
different chemical structure.
On average, triglycerides ("triacylglycerols" in
biochemistry textbooks) from fish contain 1 EPA or DHA
and 2 other fatty acids. The EPA+DHA concentration of
simple extracts of fish, i.e. fish oils, does,
therefore, not exceed one third on average. A high
concentration can be achieved by breaking up the
triglyceride structure via transesterification with
ethanol leading to ethyl esters. On a small scale, the
transesterification is
used for preparing fatty acid methyl esters for gas
chromatography. On the right: the ethyl ester of EPA which
is a major (48%) component of Omacor
Ethyl esters provide a retarded
and sustained uptake of EPA and DHA in the
duodenum
Whether the free EPA and DHA level is raised
sufficiently for antiarrhythmic action, depends not
only on the fatty acid release from membrane
phospholipids involving particularly phospholipase A2
but also on the absorption of orally administered EPA
and DHA. In fish, EPA and DHA occur as triglycerides.
Regular fish oils contain up to 30% EPA+DHA
triglycerides. If a once daily administration of one
capsule is required for achieving an intake of 1g
EPA+DHA, Omacor has to be used. Triglycerides are
transesterified with ethanol resulting in a mixture of
saturated and unsaturated ethyl esters. After
purification, nearly homogeneous EPA and DHA ethyl
esters can be prepared (Omacor
/ Lovaza). The corresponding ethyl esters should
not be referred to as fish oils simply because they
contain EPA and DHA. They are also not 'refined' or
'concentrated' fish oils. It is thus unscientific to
refer to the omega-3 preparation used in the trials of
the GISSI group as "cheap and simple fish oil". There
is also no evidence available that Omacor can be
substituted with fish oils. This point should be made
clear in guidelines. Fish oil is also not a "generic"
of Omacor.
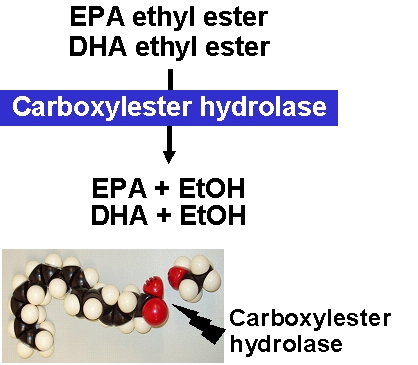
The type of ester bond has important consequences for
the absorption kinetics of EPA and DHA. Duodenal
uptake rates differ between triglycerides and ethyl
esters. Triglycerides are rapidly degraded by
pancreatic lipase and, in the case of polyunsaturated
fatty acids, by carboxylester hydrolase. Compared with
triglycerides, the ethyl esters of EPA and DHA are
absorbed more slowly. This has been shown in rats when
EPA and DHA were administered by gavage either as
triglycerides or ethyl esters (2).
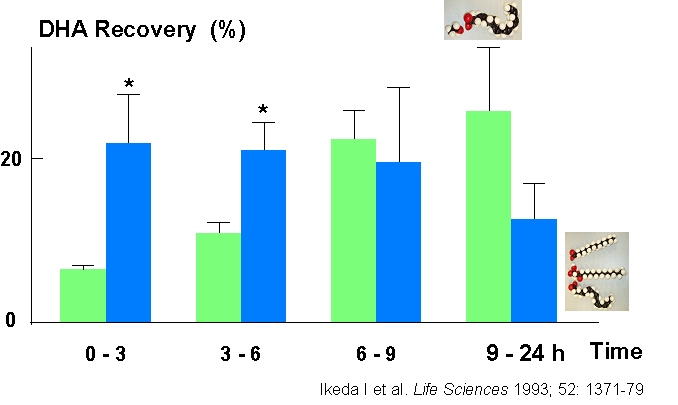
Within
3
h
after administration, the recovery in the lymph of the
respective fatty acids was greater in the case of
triglycerides (2).
After
15
h,
the recovery from ethyl esters was approximately
doubled compared with triglycerides. One of the
consequences is that the lymph EPA and DHA level is
maintained at a higher level in the second half of a
24-h period which could be of importance, since
malignant ventricular arrhythmias are more abundant in
the early morning hours (3).
Lymph
EPA
and
DHA levels arising from fish consumption during the
preceding day would thus be expected to be lower than
in the case of an ethyl ester administration. The
different absorption kinetics seen in the rat appear
to hold also for humans. Thus, the recovery of EPA in
the blood was lower within an 8-h period when compared
with triglycerides (4),
while
there
was
no difference in the long-term incorporation of EPA
and DHA involving time periods up to 28 days (5).
Although the absorption of ethyl esters is increased
by co-ingestion with a high-fat meal, the absorption
of EPA ethyl ester was still lower (6).
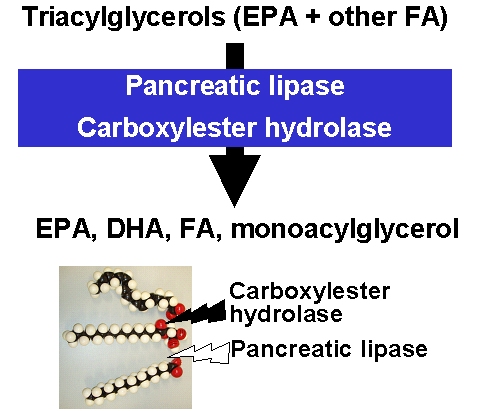
Ethyl esters
are taken up more slowly than triglycerides but
nonetheless are absorbed within 24-h to the same
extent as triglycerides.
The slowed uptake of ethyl esters
results in a "dual mechanism of action" of EPA and
DHA:
1. Sustained uptake
A sustained uptake of EPA+DHA into blood which is
expected to be beneficial in ischemic events before
EPA+DHA can be released. This could be crucial, since
in the case of severe arrhythmias there is little time
left for the release of fatty acids from tissue
stores. EPA+DHA should already be in the blood. In contrast to
EPA and DHA ethyl esters, EPA and DHA
triglycerides present in fish are more rapidly
absorbed and are expected to provide less protection
in the early morning hours when the risk of sudden
death is high and the triglycerides were consumed the
day before. The slowed recovery of ethyl esters in the
lymph has been shown by Ikeda et al. (2)
in
rats
where
fatty acids were either given as triglycerides or
ethyl esters.
These data
suggest, therefore, that the "retard" formulation of
ethyl esters has the advantage of providing increased
non-membrane bound EPA and DHA levels which are
expected to contribute to the critical rise in EPA and
DHA required for an antiarrhythmogenic action. In the
case of EPA and DHA triglycerides, a greater amount
had to be released from membranes by ischemic events.
These
considerations are based on a once daily
administration which is relevant in patients after MI
being on standard therapy with beta-blocker, ACE
inhibitor, anti-platelet drug and statin or patients
with HF on beta-blocker, ACE inhibitor, diuretic and
aldosterone antagonist.
2. Increased tissue stores
An increased EPA+DHA intake results in a higher
EPA+DHA tissue store for release during ischemic
events. The size of the tissue store is measured by
the "EPA+DHA level". The released free fatty acids EPA
and DHA are incorporated in the microenvironment of
ion channels and modulate their activity. This is
associated with an increased electrical stability as
shown by a greater refractory period or
hyperpolarisation in cardiomyocytes (10).
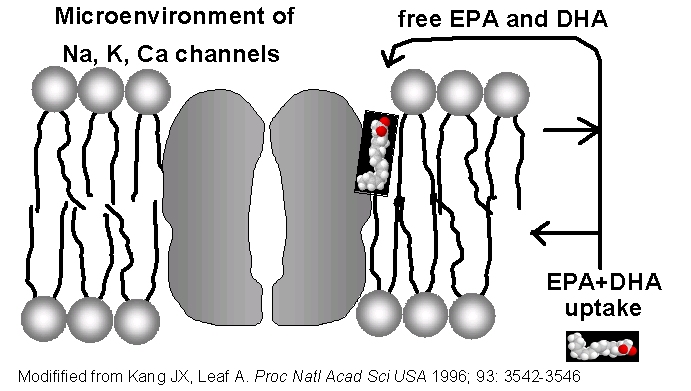
For the
administration of 1 g/day highly purified EPA+DHA
ethyl esters (Omacor
/ Lovaza) to healthy volunteers, it has been
shown that whole blood EPA is increased from 0.6% to
1.4% within 10 days while DHA is increased from 2.9%
to 4.3%. After withdrawal, EPA and DHA approach
baseline values within 10 days (data from Rupp et al.
(1)).
A
gas
chromatographic
procedure was established which requires only 10 µl of
whole blood for the identification of more than 35
fatty acids. A gas chromatogram of Omacor
demonstrating its high purity is given here.
A low “EPA+DHA level”
represents a risk for sudden cardiac death
The important study by CM Albert et al (11)
showed that low whole blood levels of the long-chain
omega-3 fatty acids EPA (C20:5n-3) and DHA (C22:6n-3)
but not of the long-chain docosapentaenoic acid
(C22:5n-3) and not of the short-chain alpha-linolenic acid
(C18:3n-3) were associated with an increased risk of
sudden death:
Fatty acid
|
GROUP WITH SUDDEN DEATH
FROM CARDIAC CAUSES
|
CONTROL GROUP
|
P VALUE
|
EPA
(eicosapentaenoic acid), long-chain n-3
|
1.72±0.59
|
1.84±0.53
|
0.06
|
DHA
(docosahexaenoic acid), long-chain n-3
|
2.12±0.65
|
2.38±0.78
|
0.005
|
DPA (docosapentaenoic acid),
long-chain n-3
|
0.98±0.23
|
1.01±0.21
|
0.25
|
alpha-Linolenic acid, short-chain
n-3
|
0.39±0.16
|
0.37±0.15
|
0.28
|
Based on this study, we proposed the term “EPA+DHA level” for
assessing the risk of sudden death (1).
In healthy
volunteers, administration of 840 mg/day of EPA+DHA
ethyl esters (Omacor
/ Lovaza) raised the “EPA+DHA level” in
whole blood to approx. 6% (1). In the GISS-P trial, this dose of
EPA and DHA ethyl esters was associated with a
marked protection from sudden death.
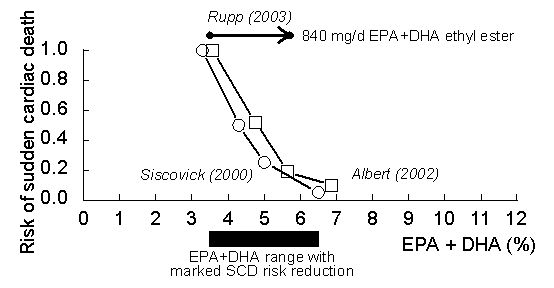
Interrelationship between the
EPA+DHA level and risk of SCD. Data are adapted
from the epidemiological studies of (open squares)
Albert et al. (11)
and (open circles) Siscovick et al. (12).
The
data
of
Albert et al. (11)
include also docosapentaenoic acid and are,
therefore, by estimated 0.98 percentage points
higher than in our study where docosapentaenoic
acid was not included. As in our study involving
840 mg/d EPA+DHA ethyl ester (Omacor
/ Lovaza) administration, whole blood was
analyzed in the study of Albert et al. (11).
Data
of
Rupp
et al. (2003) are from (1).
The predicted
reduction in the risk of SCD can account in part for
the reduced mortality observed in the GISSI-Prevention
Study with 840 mg/day EPA+DHA ethyl esters (7,
8,
9). One has,
however, to take into account that the above
epidemiological studies cannot identify the mechanisms
which result in high EPA+DHA levels in particular
patients. In our opinion, certain variations occur in
the endogenous EPA+DHA production (altered activities
of the corresponding desaturases). It appears less
likely that the high EPA+DHA levels observed in the US
in a subset of the patients arise from a very high
fish intake.
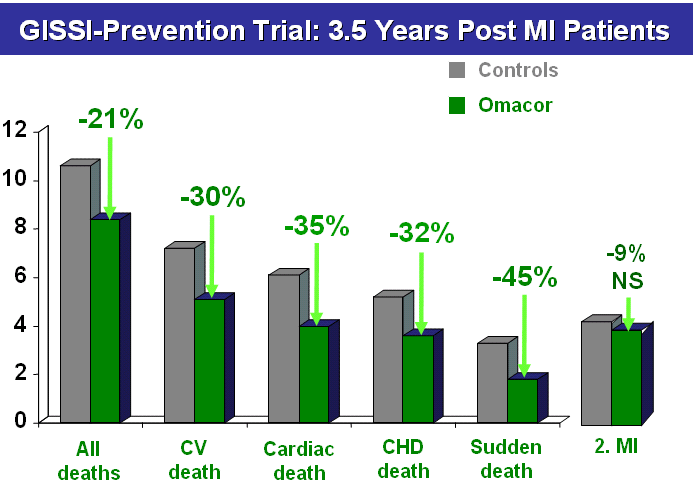
In the GISSI
Prevention (Prevenzione) Study (7,
8,
9), patients who survived a myocardial
infarction were treated with 1g/day Omacor
/ Lovaza. Mortality risk was reduced by 20%,
cardiovascular mortality risk by 30% and sudden
cardiac death risk by 45%. Since the risk
of re-infarction was not affected by Omacor
/ Lovaza, it appears that the EPA+DHA
ethyl esters had a specific action on mechanisms
leading to SCD, i.e. an anti-arrhythmogenic
action. The patients received standard care
(anti-platelet drug, beta-blocker, ACE-inhibitor
and at the end of the study also a statin).
Noteworthy is that the patients consumed on
average approx. 1 fish meal per week and that
Omacor exhibited a protection on top of the
dietary fish intake.
Further support for
antiarrhythmogenic effects of omega-3 fatty acids was
provided in the study of Calo et al. (14).
Two
1
g
capsules of EPA and DHA ethyl esters were administered
during hospitalization in patients undergoing coronary
artery bypass graft surgery (CABG). Postoperative
atrial fibrillation developed in 27 patients of the
control group (33.3%) and in 12 patients of the EPA
and DHA ethyl ester group (15.2%) (P = 0.013). There
was no significant difference in the incidence of
nonfatal postoperative complications, and
postoperative mortality was similar in the EPA and DHA
ethyl ester treated patients (1.3%) versus controls
(2.5%). After CABG, the EPA and DHA ethyl ester
treated patients were hospitalized for significantly
fewer days than controls (7.3 ± 2.1 days vs. 8.2 ± 2.6
days, P = 0.017).
In view of the
present evidence, it is suggested to include the
determination of fatty acid profile in the list of
investigated parameters in patients with
cardiovascular disease, particularly in patients after
MI. This would strengthen the rationale of therapeutic
regimens with EPA and DHA ethyl esters, as specified
in current guidelines (15,16).
Since
only
10
µl of whole blood are required, it does rarely require
additional blood sampling. By monitoring the EPA+DHA
level, patients could be identified who are at an
increased risk of SCD irrespective of their underlying
disease. Furthermore, longitudinal changes in the
EPA+DHA incorporation can be monitored and it can thus
be assessed whether a required EPA+DHA level has been
reached.
For
reducing pro-inflammatory eicosanoids and cytokines, a
higher “EPA+DHA level” is required which can be
achieved with an intake of 2 - 4 g/day of 84% EPA+DHA
ethyl esters. For assessing influences from
pro-inflammatory eicosanoids and cytokines, the
EPA/arachidonic acid ratio (“EPA/AA ratio”) appears as
a useful diagnostic parameter and deserves further
investigation
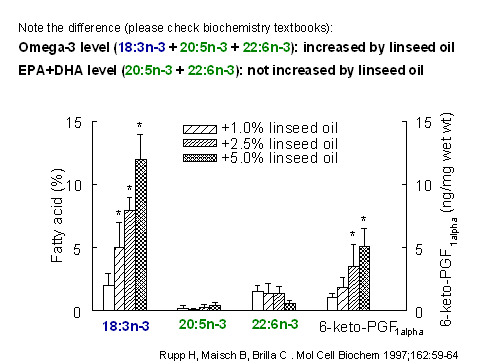
Avoid misconceptions:
EPA+DHA level and Omega-3
level are not the same
If we
want to specify the percentage of the long-chain
omega-3 fatty acids EPA and DHA, then we use the
term EPA+DHA level. Why do we not use the term
"Omega-3 level"? There is a simple answer: omega-3
includes also other omega-3 fatty acids. There are
conditions where the Omega-3 level changes not,
however, the EPA+DHA level. In one of our
experiments, rats were fed linseed oil which is
rich in the omega-3 alpha-linolenic acid. The
Omega-3 level markedly increased, but not the
EPA+DHA level. Why should we say, the omega-3
level did not increase because we (contrary to
textbook knowledge) defined omega-3 level as sum
of only EPA+DHA?
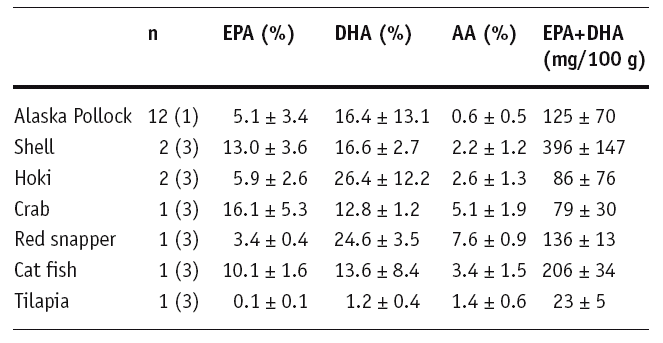
Fish meals are not a substitute for Omacor
To assess the role of dietary EPA+DHA intake, fatty
acids were determined in fish dishes of the cafeteria
of
the
Philipps
University Marburg. The EPA+DHA content of the
popular Alaska Pollock was 125±70 mg/100 g (1).
A once daily fish dish can thus not
provide the 840 mg/day EPA+DHA administered in the
GISSI trials in the form of ethyl ester which markedly
reduced the risk of SCD in post-MI patients.
Nonetheless, at least two preferably oily fish meals
per week should be consumed as preventive measure by
persons without coronary artery disease. With
documented coronary heart disease, it was advised to
consume approximately 1 g/day of EPA+DHA (16).
Fish oils
must not be substituted for Omacor
Great progress has been made in prevention of
cardiovascular diseases. A major contribution came
from randomized double blind clinical trials. Therapy
is based on the outcome of these trials and we are
privileged to live in the age of evidence-based
medicine. Why do we mention this in the context of
omega-3 fatty acids? In contrast to the nomenclature
of pharmaceuticals, the term "omega-3 fatty acids" is
often used in an unscientific manner. While in the
trials of the GISSI group Omacor was administered, the
impression is often generated that "omega-3 fatty
acids or "omega-3 PUFAs" were administered. It is
implicated that it does not matter whether Omacor or
other preparations of "omega-3 fatty acids" are used.
Incidentally, the other preparations are cheaper (less
costly to prepare because of less stringent
regulations in case of OTC nutrition supplements). So
this misconception is readily accepted. But do these
other "omega-3" preparations work? The answer is: we
don't know, there is no evidence available at all. So
in this respect we turn away from evidence-based
medicine. A patient who survived an MI should be
treated based on the outcome of the trials of the
GISSI group and not on the basis of assumptions.
Obviously, manufacturers of fish oil capsules are
invited to do clinical trials and to prove the
efficacy of their products. In view of the huge sales
of fish oils, it should not be a financial burden for
the companies to support these trials. If physicians
prescribe Omacor and pharmacists recommend
substitution with fish oils, they are - in our opinion
- in breach of their own ethical guidelines. Pharmacists must
be aware of the differences and cannot mislead
patients. Pharmacists have to give customers proper
information. The ethical code states that pharmacists
"must assist patients in making informed decisions" by
providing them with "necessary and relevant
information" (see also the recent discussion on homeopathic
remedies. Put in simple terms: fish oils are not
"generics" of Omacor. It has been argued that the level
of EPA+DHA is some type of surrogate endpoint and as
long as a specified (high) level is reached, it does
not matter how it is reached. What is the evidence for
this? There is no evidence!
"Any" brand of ethyl ester
must not be substituted for Omacor
Fish oils are
different from Omacor, so clearly they cannot be
substituted for Omacor. But what about "omega-3 ethyl
esters" in general? Again the same situation: in the
GISSI trials, Omacor was used but not "any" brand of
ethyl esters. There are indeed major differences in
the preparations. This is exemplified by the
comparison of two ethyl ester preparations, referred
to as preparation A and preparation B. Although
preparation A and preparation B contain 90% omega-3
fatty acids, preparation B contains only 20% DHA and
60% EPA while preparation A contains 38% DHA and 46%
EPA. Furthermore, by including various minor omega-3
fatty acids, the total omega-3 fatty acid content is
raised in preparation B to 90%, although the
biological role of these minor (including also
short-chain) omega-3 fatty acids remains unresolved.
Therefore, preparation B is clearly not a substitute
for preparation A. Since no clinical trials have been
done with preparation B, we consider it unethical to
come up with a substitution simply because preparation
B is cheaper than preparation A (Omacor). Again the
argument comes up that the EPA+DHA level might be a
good enough surrogate endpoint or predictor of sudden
death. Too often we have learned that predictions for
cardiovascular endpoints turned out to be wrong. A
very recent example is the lack of efficacy of a
statin in heart failure despite its pleiotropic
actions.
For an overview on the concept
of the "EPA + DHA level" as related to
cardiovascular risk,
please see:
Rupp H, Wagner D, Rupp T, Schulte L,
Maisch B. Risk stratification by the "EPA+DHA
Level" and the "EPA/AA Ratio". Focus on
anti-inflammatory and antiarrythmogenic effects
of long-chain omega-3 fatty acids. Herz 2004;
29:673-685 (available as
PDF)
Rupp H, Rupp
TP, Alter P, Maisch B. Acute Heart Failure –
Basic Pathomechanism and New Drug Targets. Herz
2006;31:727-735 (available as
PDF)
Rupp
H, Rupp TP, Wagner D, Alter P, Maisch B.
Microdetermination of fatty acids by gas
chromatography and cardiovascular risk
stratification by the "EPA+DHA level". Herz
2006;31Suppl 3:30-49 (available
as PDF)



(1)
Rupp
H,
Wagner
D, Rupp T, Schulte L, Maisch B. Risk
stratification by the "EPA+DHA Level" and the
"EPA/AA Ratio". Focus on anti-inflammatory and
antiarrythmogenic effects of long-chain omega-3
fatty acids. Herz 2004; 29:673-685.
Also available as PDF
(2)
Ikeda
I,
Imasato
Y, Nagao H, et al. Lymphatic transport of
eicosapentaenoic and docosahexaenoic acids as
triglyceride, ethyl ester and free acid, and their
effect on cholesterol transport in rats. Life Sci
1993;52:1371–9.
(3)
Kozak
M,
Krivan
L, Semrad B. Circadian variations in the
occurrence of ventricular tachyarrhythmias in
patients with implantable cardioverter
defibrillators. Pacing Clin Electrophysiol
2003;26:731–5.
(4)
Lawson
LD,
Hughes
BG. Human absorption of fish oil fatty acids as
triacylglycerols, free acids, or ethyl esters.
Biochem Biophys Res Commun 1988;152:328–35.
(5) Luley C, Wieland H, Grünwald J. Bioavailability
of omega-3 fatty acids: ethylester preparations are
as suitable as triglyceride preparations. Akt
Ernährungsmed 1990;15:123–5.
(6)
Lawson
LD,
Hughes
BG. Absorption of eicosapentaenoic acid and
docosahexaenoic acid from fish oil
triacylglycerols or fish oil ethyl esters
co-ingested with a high-fat meal. Biochem Biophys
Res Commun 1988;156:960–3.
(7)
GISSI-Prevenzione
Investigators.
Dietary
supplementation with ω-3 polyunsaturated fatty
acids and vitamin E after myocardial infarction:
results of the GISSI-Prevenzione Trial. Gruppo
Italiano per lo Studio della Sopravvivenza
nell’Infarto miocardico. Lancet 1999;354:447–55.
(8)
Marchioli
R,
Avanzini
F, Barzi F, et al. Assessment of absolute risk of
death after myocardial infarction by use of
multiple-risk-factor assessment equations:
GISSI-Prevenzione mortality risk chart. Eur Heart
J 2001;22:2085–103.
(9).
Marchioli
R,
Barzi
F, Bomba E, et al. Early protection against sudden
death by ω-3 polyunsaturated fatty acids after
myocardial infarction: time-course analysis of the
results of the Gruppo Italiano per lo Studio della
Sopravvivenza nell’Infarto Miocardico
(GISSI)-Prevenzione. Circulation 2002;105:
1897–903.
(10)
Leaf
A,
Xiao
YF, Kang JX, et al. Prevention of sudden cardiac
death by ω-3 polyunsaturated fatty acids.
Pharmacol Ther 2003;98:355–77.
(11)
Albert
CM,
Campos
H, Stampfer MJ, Ridker PM, Manson JE, Willett WC,
Ma J. Blood levels of long-chain n-3 fatty acids
and the risk of sudden death. N Engl J Med 2002;
346:1113-1118.
(12)
Siscovick
DS,
Raghunathan
TE, King I, Weinmann S, Wicklund KG, Albright J,
Bovbjerg V, Arbogast P, Smith H, Kushi LH. Dietary
intake and cell membrane levels of long-chain n-3
polyunsaturated fatty acids and the risk of
primary cardiac arrest. JAMA 1995; 274:1363-1367.
(13)
Leaf
A, Albert CM, Josephson M, Steinhaus D, Kluger J,
Kang JX, Cox B, Zhang H, Schoenfeld D. Prevention
of fatal arrhythmias in high-risk subjects by fish
oil n-3 fatty acid intake. Circulation
2005;112:2762-2768
(14)
Calo
L,
Bianconi
L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo
E, Meo A, Pandozi C, Stai-bano M, Santini M. N-3
Fatty acids for the prevention of atrial
fibrillation after coronary artery by-pass
surgery: a randomized, controlled trial. J Am Coll
Cardiol 2005;45:1723-1728.
(15)
Kris-Etherton
PM, Harris WS, Appel LJ; American Heart
Association. Nutrition Committee. Fish
consumption, fish oil, omega-3 fatty acids, and
cardiovascular disease. Circulation.
2002;106:2747-57.
(16)
Van de Werf F, Ardissino D, Betriu A, Cokkinos DV,
Falk E, Fox KA, Julian D, Lengyel M, Neumann FJ,
Ruzyllo W, Thygesen C, Underwood SR, Vahanian A,
Verheugt W, Wijns W.
Management of acute myocardial infarction in
patients presenting with ST-segment elevation. The
Task Force on the Management of Acute Myocardial
Infarction of the European Society of Cardiology.
Eur Heart J 2003;24:28-66.
|
Omega-3-Forum
"Make everything as simple as
possible, but not simpler."
Be more precise:
the
"EPA+DHA level"
Not all omega-3 fatty acids are the same (see
textbooks of biochemistry) and their protective
effects depend on the chain length and number of
double bonds. Only long-chain omega-3 fatty acids
(EPA+DHA) but not the short-chain omega-3
alpha-linolenic acid have been shown to reduce risk
of sudden death (Albert
CM et al.).
If e.g. the "omega-3 level" is calculated, obviously
also the omega-3 alpha-linolenic acid has to be
included (or you redefine what you mean with omega-3
and simply ignore textbook knowledge). Thus, terms
like "omega-3 level" are too broad and not
appropriate when referring to benefits observed in
the GISSI-Prevention and GISSI-HF trials.
There is also a discrepancy between the design of
well-controlled trials and the inprecise
specification of the medication used. To our
knowledge, the ratio of DHA:EPA in the GISSI trials
was 1: 1.2 and not the reverse. This ratio is found
in Omacor, i.e. 38% DHA and 46% EPA. These
prescription omega-3 fatty acid ethyl esters
must not be referred to as "cheap and simple fish
oil" and also not as "highly purified fish oil".
Fish oil contains triglycerides whereas Omacor
contains ethyl esters. Triglycerides but not ethyl
esters are split by pancreatic lipase and thus
rapidly absorbed. Ethyl esters result in a retarded
sustained EPA and DHA absorption. It should not be
tolerated that the public is misled in this respect.
Recent questions:
Monitoring beyond omega-3 fatty acids?
The arachidonic acid:EPA ratio needs to be
re-evaluated.
Gas chromatography
How to work safely with hydrogen: use a hydrogen
generator.
Sample preparation and
standardization
Use alkaline conditions in the transesterification
with methanol. The typical BF3/method results in a
loss of EPA and DHA. Details are given in Rupp
et al.
Alternative tests
Are the "fast" GC methods an alternative?
Other sites maintained by us:
www.cleverfood.com
www.cardiorepair.com
www.carditis.com
www.herzzentrum-marburg.de
herzzentrum.online.uni-marburg.de
|